PACS 31; 32.30.Rj
UDC 539.26
A.T. Serkov, A.A. Serkov
SIC Ltd. (Research and Engineering Center) "Uglehimvolokno" 141,009. Moscow region. Mytischi str. Kolontsova, 5. e-mail: arkady07@rambler.ru
The behavior of the electrons flow in the crystal lattice subjected to a microgravity forces from the atoms forming the crystalline lattice is considered. Electrons under the influence of microgravity forces change its trajectory (orbit) of flight. Depending on the distance of an electron from the atom core the electron orbit becomes elliptical, parabolic, and hyperbolic or remains straight. The scattering occurs electron flow is macroscopically perceived as diffraction on the lattice. Consequently, the "wave" properties of electrons are explained within the framework of the ordinary mechanics of the orbital interaction of particles with a constant value in the law of gravitation 1.847·1028 cm3 / gc2 and can not be an experimental basis for wave mechanics.
A Discovery de Broglie wave played a major role in quantum mechanics and was the basis for the development of one of its main sections - wave mechanics. De Broglie suggested that the moving particle with energy E and velocity v, is characterized by some internal periodic process with frequency E/h = ν, where h - Planck's constant Any moving particle (e.g., electron) behaves not only as a spatially localized moving object (corpuscle), but also as a wave. Wavelength of the particle is defined by λ = h/p, where p - momentum of the particle. If the particle has mass m and velocity v, the particle momentum p = mv and the de Broglie wavelength are related by:
λ = h/mv. (1)
The existence of de Broglie waves is proved by numerous experiments in which particles behave like waves. So in the scattering of the electron beam with energy of 100 eV for the ordered system of atoms in a crystal, which acts as a diffraction grating, there is a distinct diffraction pattern. Having de Broglie waves underlies of electron microscope resolving power which is many orders of magnitude higher than that of an optical microscope that allows one to observe atoms and molecules. Particle diffraction method is now widely used to study the structure and properties of the substance.
This phenomenon of the dual behavior of the electron as a particle and as a wave in physics received the title as wave-particle duality. In conjunction with the uncertainty principle dualism electron behavior is the basis of modern description of the structure and functioning of the atom. That picture of the atom as given in this case: "If the hydrogen atom in the lowest energy state could take a picture with a slow shutter speed, we would see an electron cloud with variable density. Electron can be detected with different probabilities at any point in the cloud”. This approach to the nuclear issue is a significant part of the dissatisfaction of researchers. There is a clear public "order" for the deterministic approach. In this message is an attempt to fill the need for this dissatisfaction.
Previously [1] hypothesis P. Laplace "modification of the gravitational forces in the molecular" by increasing density of the micro particles substance to 1012 g/cm3 was confirmed. Nucleus and electrons in the atom interact in the inverse square of microgravity law, where the constant in 1036 times more Newtonian constant and equal to 1,845·1028 sm3/gs2. Unusually large microgravity constant value causes a very large value of attraction forces that act on an extremely small distances. This leads to the same phenomenon as diffraction of light. The flow of electrons interacting with atomic nuclei, scattered, and the scattering pattern depends on the arrangement of atoms, electrons flow rate and the interaction forces between atoms and electrons. Thus, it is possible to explain the wave properties of electrons on the base of microgravity particle interaction involving the laws of dynamics orbital motion without resorting to wave representations and wave mechanics.
Microgravity interaction of electrons flow with atoms of the crystal lattice is expressed by microgravity formula, which is similar to Newton's law of gravity equation:
f = gmema/r2, (2)
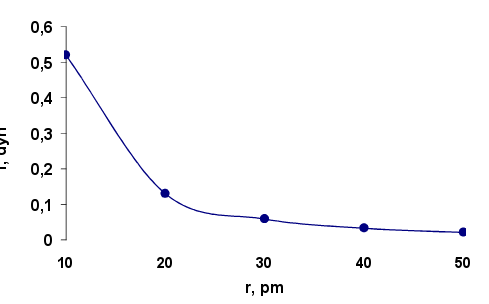
where f-interaction force, g- microgravity constant, me and ma - respectively the mass of the electron and the nucleus of the atom, r-distance between the atoms in the crystal lattice. Estimation of the interaction force of electrons located at different distances from the atom nucleus is made for a crystalline nickel, which was first shown electron diffraction. Dependence of the attraction force of the electrons by nucleus of nickel atom from of atom radius is shown in Figure 1. Calculation of the force f is made to equation (2) at the microgravity constant 1,845·1028 sm3/gs2. Interplanar spacing in the crystal Ni equals 90.9 pm. Atomic radius according to published data 124 pm. The atomic mass is 58.69. Electron mass is taken to be 0,294·10-24g.

Fig.1. Dependence of the attraction electron force f by a nucleus of nickel atom on the radius
The calculations show that in the inner part of the nickel atom at a distance of 10 pm from the nucleus, the electron experiences a strong enough attraction equal to 0.53 diners. With increasing radius the attractive force decreases quadratically and equidistant distance from Ni atoms in the crystal lattice is only 0,026 diners, i.e. falls 20 times. Such a strong decrease in the force of electron attraction by the core at short distances leads to a large gradient force interaction and the strong scattering of electron flow. The degree of scattering can be determined using the equations of the dynamics of the orbital motion of electrons under the influence of the microgravity attraction force of the atomic nuclei.
In the case where the electron kinetic energy is equal to the potential energy, the electron is captured by the microgravity force of attraction from the nucleus of an atom core and removed from the flow. This is an extreme case of scattering. The sharp decrease in the force of attraction is observed with increasing distance from the nucleus at 20-25 pm. Increasing the distance from the core leads to a decrease in the force of attraction and the transition to the motion on the less curved parabolic, then hyperbolic trajectory. The scattering of electron flow decreases. Finally, at an equal distance from the atoms of the crystal lattice is no scattering.
The microgravity interaction force causes a deviation from the rectilinear motion of the electrons, which is similar to the phenomenon of diffraction of light; this deviation can be considered as electron diffraction. Scheme of interaction flow of electrons with atoms of the crystal lattice is shown in Figure 2.
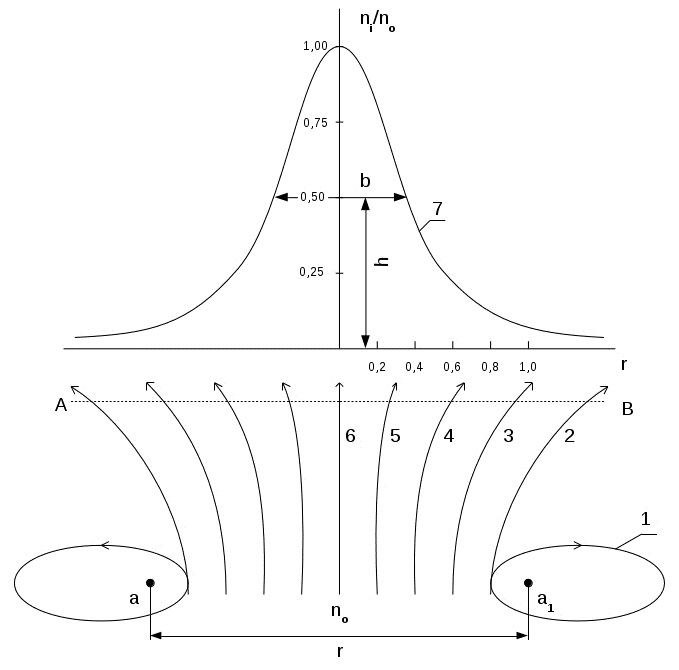
Fig.2. Scheme of interaction of electrons flow with atoms of the crystal lattice:
n0 - flow of electrons, a and a1- atoms of crystal lattice , r- distance between atoms , 1 - elliptical orbit , 2 - parabolic orbit , 3.4 and 5 - hyperbolic orbits , 6 - initial rectilinear trajectory flight electron flow, 7 - flow density distribution curve of the electrons depending on a distance between atoms ni/n0 - relative density of electrons in the stream , h- half peak height maximum density , b- width of the peak of the distribution curve at half maximum of the peak density.
The flow of electrons density n0 moves between atoms a and a1 of the crystal lattice, located at the distance r from each other. Depending on the initial velocity and the distance from the atom, electrons can move along the closed elliptical orbit (1) open parabolic (2), a hyperbolic (3,4,5) or straight (6) orbits.
The flow of electrons density n0 moves between atoms of the crystal lattice a and a1, located at the distance r from each other. Depending on the initial velocity and the distance from the atom, electrons can move along the closed elliptical (1) open parabolic (2), a hyperbolic (3,4,5) or straight (6) orbits. Deviation of the electron trajectory from the initial rectilinear direction leads to their scattering, which is shown in the figure curve (7). It expresses the dependence of the relative electron density ni/n0 on the distance between the atoms of the crystal lattice. By analogy with the X-ray approach the degree of electrons scattering will characterize by the relative peak width b at half its height h, i.e. b / h. This value is substantially similar to the wavelength, as characterizes the linear dimension of the electron beam and the width of the reflex formed on the screen. Linear dimension of the beam, in turn, determines the resolution of the electron microscope. Resolution, as is known, depends on wavelength for an electron microscope at 1000 times the resolution of the optical microscope.
In accordance with equation (1) The wavelength of flying particle decreases with increasing the mass and the particle velocity. In this case, increasing the mass and velocity leads to a decrease in the ratio b / h, respectively, and to increase the resolution, this is consistent with the findings of de Broglie’s theory and experimental data. At the same time, the phenomenon of electron diffraction is considered without involving wave theory, and based on classical physical laws, in particular the dynamics of orbital motion. A necessary and sufficient condition for such an approach to solving the problem is to take as valid hypothesis P. Laplace "modification of the gravitational forces in the molecular" when the density of matter in elementary particles up to 1012 g/cm3.
However, de Broglie's hypothesis that "moving particle with energy E and velocity v, is characterized by some internal periodic process" has a meaning if the two parameters E and v, add a third - force f, through movement. The diagram in Figure 2 reflects the two extreme cases. In the first case, when the electron is equidistant from atoms and attractive forces are balanced, it moves in a straight line and no periodicity in its movement observed.
In the second extreme case, when the potential energy of attraction and the kinetic energy of a moving body or the force of attraction and the power of inertia are equal, then there is a radical change in traffic patterns. It begins to be carried out on an elliptical or circular orbit, i.e. it becomes periodic oscillatory. But of course this does not mean that a moving particle has turned into a wave. It simply started to carry oscillating movement.
This phenomenon can be transferred to macro objects. Any moving macro body (satellites, moon, planet) is inherent possession of "internal periodic process" if the condition of the transition from the translational motion of to orbital, i.e. the cycle of oscillatory motion, is realized
Ek = Ep = M2v2/2 = GM1M2 / R or v2 = 2GM1/R, (3)
where Ek and Ep-kinetic and potential energy of the body, M1 and M2 is the mass of the central and orbital body, v-orbital velocity of the body, R-distance between the bodies. In case a macro bodies when calculating the oscillatory motion is taken into account the gravitational constant of course.
1. It is calculated the microgravity attraction force for electron by nickel atoms in the crystal lattice. At a distance of 10 pm from the nucleus of an atom it is 0.53 dynes. As the distance increase the force decreases quadratically and at the same distance from the Ni atoms is 0,026 dynes. Large gradient of the microgravity attractive force leads to a significant deviation of the trajectory of the electrons and their scattering, which macroscopically perceived as electron diffraction.
2. Electron diffraction can be explained on the basis of the classical laws of physics, if we accept the hypothesis P. Laplace adequacy "modification of the gravitational forces in the molecular" by increasing the density of matter in elementary particles up to 1012 g/cm3.
3. The theory of particle-wave dualism and wave mechanics are not supported by the phenomenon of electron diffraction, which is explained in the framework of classical physical laws of gravitation and orbital motion.
1. A.T. Serkov, A.A. Serkov, Microgravity, electricity, forces of attraction and repulsion in the atom.